Orange County is home to some of the hottest medical device and biotechnology companies, producing a range of drugs and devices that address health issues affecting millions of people every day – from diabetes to incontinence to blood clots in veins.
The life science industry employed more than 60,000 workers in OC and generated $7.5 billion in labor outcome and output of $45 billion, according to Biocom California’s Life Science Economic Impact Report.
They report that medical devices and equipment were the largest sub-sector of life science employment, accounting for 42%.
What follows is a breakdown of 10 notable medtech and biomed companies (see related list, page 28) in OC and their most well-known products.
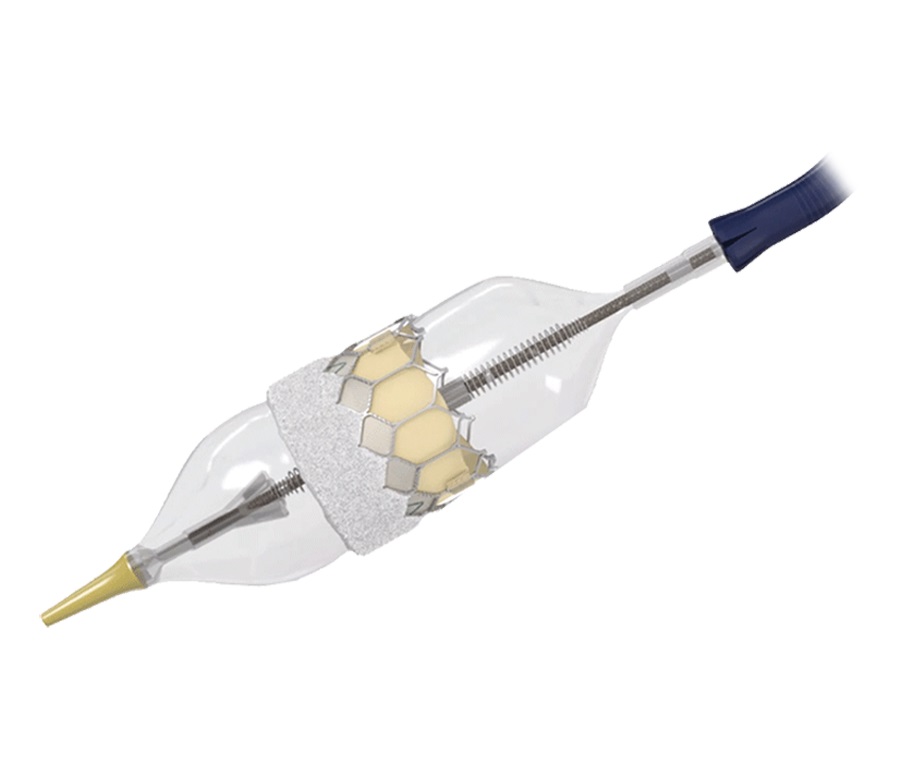
Edwards Lifesciences Corp.
• FOUNDED: 1958
• MAIN PRODUCT: Sapien transcatheter aortic heart valve
One of the world’s leading medical device companies is located right here in our backyard.
Edwards Lifesciences Corp. (NYSE: EW) is the second largest public company in Orange County with a market cap of $41.6 billion, second only behind Chipotle Mexican Grill Inc.
The Irvine-based company was acquired by Baxter International Inc. in 1985, then spun out in 2000. It was named in honor of Miles “Lowell” Edwards, who built the world’s first mitral valve successfully placed in a patient.
Edwards’ breakthrough product came in the form of an aortic heart valve that could be implanted without the need for open-heart surgery.
In 2004, Edwards paid $155 million to acquire PVT, a company experimenting with a catheter to replace aortic heart valves. Instead of performing open heart surgeries, a catheter could be introduced in a small hole in the upper thigh and make its way to a diseased heart valve. The replacement valve would then inflate like a balloon, pushing aside the diseased valve.
The procedure was approved by the Food and Drug Administration in 2011. It’s now the company’s largest business unit, known as Transcatheter Aortic Valve Replacement, and accounted for $4.1 billion, which was 76% of Edwards’ sales in 2024.
Edwards’ Sapien is said to be the most-used TAVR heart valves in the industry and have been implanted in nearly 800,000 patients, according to Edwards.
—Yuika Yoshida
Beta Bionics Inc.
• FOUNDED: 2015
• MAIN PRODUCT: iLet Bionic Pancreas
A diabetes diagnosis was the inspiration behind Beta Bionics Inc.’s concept of an artificial pancreas that regulates blood sugar levels in people with Type 1 diabetes, (T1D).
The Irvine-based company, co-founded by Boston University biomedical engineering professor Edward “Ed” Damiano, took its idea public in January after raising an upsized $234.6 million initial public offering, $120 million more than planned.
Beta Bionics (Nasdaq: BBNX) launched the iLet Bionic Pancreas in late 2023 after it received Food and Drug Administration clearance for use in individuals ages six and older with T1D. It’s the first FDA approved pump to utilize adaptive learning algorithms to learn each person’s unique and ever-changing insulin requirements, according to the company.
“All of the algorithms simply react to your blood sugar and react based on pre-programmed settings,” Chief Executive Sean Saint told the Business Journal in March. “We added a learning layer above that.”
The iLet requires only one input—the user’s weight—and calculates insulin doses every five minutes throughout the day and night.
Analysts are expecting revenue to jump 27% this year to $83 million.
—Yuika Yoshida
Axonics Inc.
• FOUNDED: 2012
• MAIN PRODUCT: Axonics Therapy system
Axonics Inc.’s vision of neurostimulation playing a big role in medical therapy came to fruition a little over a decade after it was founded. Last November, Boston Scientific Corp. announced that it closed on the $3.7 billion acquisition of the Irvine-based medical device maker that specializes in urology.
The acquisition was announced Jan. 8, 2024, the same day that the Business Journal named Axonics Chief Executive Raymond Cohen Businessperson of the Year in the healthcare sector.
Cohen co-founded the company in 2012 and took it public in 2018. Analysts are expecting revenue to jump 27% this year to $83 million. It became one of Wall Street’s fastest growing companies for several years. Its sales in 2023 grew 34% to $366 million.
Axonics’ offers two implantable devices that help with overactive bladders, an issue that affects an estimated 87 million adults in the U.S. and Europe, according to the company.
The devices send electrical signals to the sacral nerve to help regulate the bladder and bowels.
Since the close of the acquisition, Axonics’ sacral neuromodulation systems have been added to Boston Scientific’s urology business.
—Yuika Yoshida
Inari Medical Inc.
• FOUNDED: 2013
• MAIN PRODUCT: FlowTriever, ClotTriever systems
Irvine-based Inari Medical Inc. is the latest success story to come out of Orange County.
The company in February was acquired by global medical technology company Stryker Corp. for $4.9 billion, one of the largest medtech acquisitions in OC in recent years.
Inari has a portfolio of medical devices and accessories to treat venous thromboembolism (VTE), or blood clots in the veins. VTE affects more than 900,000 patients yearly.
In 2018, Inari received Food and Drug Administration clearance for its FlowTriever and ClotTriever products.
Its sales climbed 29% to $494 million in 2023.
Both procedures begin with a small incision, most commonly in the leg. The short hollow tube of the device is then inserted into the vein so the catheters can reach the blood clot, where the physician uses suction to remove it.
—Yuika Yoshida
Glaukos Corp.
• FOUNDED: 1998
• MAIN PRODUCT: iStent
Glaukos Corp. (NYSE: GKOS) is the maker of the world’s smallest medical device.
The Aliso Viejo-based company, founded in 1998, pioneered the now well-established microinvasive glaucoma surgery marketplace.
Its best-known product is the iStent, a microscopic implantable device intended to reduce intraocular pressure in the eye.
Trials on the iStent began in 2005, and it won Food and Drug Administration approval in 2011, followed by a smaller iStent in 2017. The success of the tiny but mighty product is reflected in Glaukos’ $5.3 billion market cap, making it the most valuable public company in OC’s ophthalmology sector made up of device makers, drug companies and lens manufacturers.
Its newest product, iDose TR, received approval in late 2023. Once implanted, iDose secretes a formula that’s said to be 25,000 times more concentrated than typical glaucoma medication.
The company’s sales are expected to grow 25% to $480 million this year.
—Yuika Yoshida
RxSight Inc.
• FOUNDED: 1997
• MAIN PRODUCT: Light Adjustable Lens (LAL/LAL+)
RxSight Inc. (Nasdaq: RXST) has made it possible to fine-tune patients’ vision post-cataract surgery.
The Aliso Viejo company developed the world’s first and only eye lenses that can be adjusted using light technology after their cataract has been removed.
In a typical cataract surgery, the natural lens that has become cloudy is replaced with a man-made lens that is adjusted to the person’s sight before the surgery. After the surgery, if the patient still cannot see well enough, prescription glasses are ordered.
With RxSight’s Light Adjustable Lenses (LAL), doctors can change the shape and prescription of the new lens after surgery if needed.
Last April, RxSight launched the LAL+.
The lens, designed to extend the depth of focus, is fully rolled out in the U.S. and recently gained approval in Canada.
The LAL+ delivers an average of 1.3 additional lines of distance-corrected near vision with 90% of patients achieving distance vision of 20/20 or better without glasses, according to data the company presented at the American Academy of Ophthalmology meeting last October.
Analysts expect its sales to climb 22% this year to $170 million.
—Yuika Yoshida
Tarsus Pharmaceuticals Inc.
• FOUNDED: 2016
• MAIN PRODUCT: Xdemvy eyedrops
Tarsus Pharmaceuticals Inc. (Nasdaq: TARS) creates drugs treatments for one of the less pleasant ailments on this list – crusty eyelids.
The company’s eyedrop, Xdemvy, targets Demodex blepharitis, an ocular disease where mites infest the eyelid, causing inflammation and dryness among other problems around the eyes.
The ocular disease affects some 25 million Americans and had no approved treatment prior to Xdemvy, which received clearance in 2023. Since commercial launch, Tarsus has dispensed more than 163,000 bottles to patients and generated $180.1 million in net product sales.
The Irvine-based biomed firm last month closed on an upsized $134.8 million public offering. It said it plans to use proceeds to boost commercialization of Xdemvy in the U.S., as well as develop treatments for other diseases such as ocular rosacea and Lyme disease.
—Yuika Yoshida
Masimo Corp.
• FOUNDED: 1989
• MAIN PRODUCT: Noninvasive patient monitoring devices
Masimo Corp., the fifth-largest public company in Orange County, was started in founder Joe Kiani’s Aliso Viejo garage in 1989.
The company, which went public in 2007, is best-known for making noninvasive patient monitoring devices, an industry with a total addressable market around $50 billion.
The Irvine-based company’s products became famous for reducing faulty readings on the oxygen levels of newborn babies, helping reduce deaths and blindness. The devices, primarily sold to hospitals, leapt in popularity during the COVID-19 pandemic because of their ability to remotely monitor patients.
Masimo (Nasdaq: MASI) in 2022 expanded into the consumer heath market with its $1 billion acquisition of Sound United, a decision that caused the company’s market cap to fall $5.2 billion in one day and caught the attention of activist investor Politan Capital Management.
Since then, massive upheavals have taken place at Masimo, including a six month-long proxy battle with Politan that resulted in Kiani being ousted from the board last September. Kiani resigned as CEO soon after and has since been succeeded by Edwards Lifesciences veteran Katie Szyman.
Masimo last November announced that it is shifting away from investing in wearables such as Bridge and Opioid Halo amid the company’s potential spin off or sale of its consumer business.
—Yuika Yoshida
CathWorks
• FOUNDED: 2013
• MAIN PRODUCT: FFRangio System
CathWorks’ diagnostic technology has caught the attention of Medtronic plc, one of the world’s largest medical device makers.
The Newport Beach medical device company in 2022 reached a $75 million funding deal with Medtronic and has since entered into an agreement that gives Medtronic the option to buy CathWorks in the next three years at a $585 million valuation.
The company’s product is dubbed the FFRangio System.
It’s a noninvasive method of diagnosing heart disease. In just five minutes, the system can create a 3D model of a patients’ arteries using artificial intelligence and machine learning.
The FFRangio System is available in 45 U.S. states, more than over 200 hospitals in Japan and most recently gained clearance in the European Union.
CathWorks’ worldwide expansion is supported by its co-promotion partnership with Medtronic.
—Yuika Yoshida
Allergan plc
• FOUNDED: 1948
• MAIN PRODUCT: Botox
Allergan plc is the company behind well-known wrinkle-removing toxin, Botox.
Since its inception, the product has continued to dominate the market with Allergan’s aesthetics division generating $5.2 billion in sales in 2024.
Besides Botox, Allergan’s known for its portfolio of facial injectables, body contouring and skin care products.
In 2020, Allergan was bought by Chicago-based AbbVie Inc. (NYSE: ABBV) for nearly $63 billion.
The company has its Allergan Aesthetics unit based in Irvine (see more, page 1).
Allergan’s founder, Gavin Herbert, is known as the father of Orange County’s aesthetic, ophthalmic and drug making industries.
UC Irvine in 2013 opened the Gavin Herbert Eye Institute, which has become one of the nation’s biggest ophthalmology research institutes.
—Yuika Yoshida
